Nuclear Gamma Resonance (Mössbauer) Spectrosopy
 Nuclear Gamma Resonance (NGR) relies on the resonant emission and absorption of γ-ray photons by atomic nuclei in a solid.
In spite of the large energies of the γ-rays involved, these processess occur without an energy loss due to nuclear recoil,
that is, they are recoiless. This is known as the
Mössbauer effect.
The energies of the nuclear levels are perturbed by the interaction of a nucleus with its environment.
Since a nucleus is surronunded by electrons its environment is known as the electronic structure of that site.
By measuring these small environment-induced changes in the energies of nuclear levels, this technique allows us to
use of atomic nuclei as probes of their immediate molecular environment. In other words we can use nuclei as spies
to look at molecules from the inside out. Among all Mössbauer-active elements, owing to its biological
and technological importance as well as relative ease with which the experimental spectra can be collected, Iron
is the most intensively investigated.
Nuclear Gamma Resonance (NGR) relies on the resonant emission and absorption of γ-ray photons by atomic nuclei in a solid.
In spite of the large energies of the γ-rays involved, these processess occur without an energy loss due to nuclear recoil,
that is, they are recoiless. This is known as the
Mössbauer effect.
The energies of the nuclear levels are perturbed by the interaction of a nucleus with its environment.
Since a nucleus is surronunded by electrons its environment is known as the electronic structure of that site.
By measuring these small environment-induced changes in the energies of nuclear levels, this technique allows us to
use of atomic nuclei as probes of their immediate molecular environment. In other words we can use nuclei as spies
to look at molecules from the inside out. Among all Mössbauer-active elements, owing to its biological
and technological importance as well as relative ease with which the experimental spectra can be collected, Iron
is the most intensively investigated.
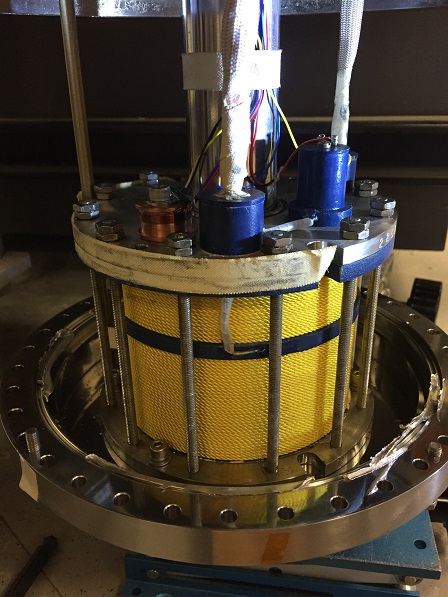 Field-dependent Mössbauer spectroscopy
is a specialized, highly powerful spectroscopic tool
that can provide a wealth of information such the oxidation state, the asymmetry of the electronic charge distribution
(electric field gradient), the relative energies of the spin sublevels of the ground state (zero-field splitting), and
the strength of the interaction between the 57Fe nuclear spins and the electronic spins (hyperfine structure) of all
iron sites contained by a chemical species of interest. This information can be extracted regardless of the nature
of the iron-containing species, that is, there are no Mössbauer-silent iron sites. Albeit the ability to only detect
a single isotope, in this case 57Fe, might seem a limitation, the widespread distribution of iron-containing enzymes
and the tremendous technological importance of this element makes Mössbauer spectroscopy the preeminent technique for
the characterization of iron species. An important feature of this technique is that it allows for the quantification
of all iron species in a sample. However, this analysis requires recording zero-field spectra at very low temperatures,
usually below 10 K. At higher temperatures the fraction of nuclei that exhibit a recoilless absorption and emission of
γ-ray photons might be different for different sites. Performing field- and temperature-dependent measurements
informs us on the magnetic behavior of all iron species present in the sample, that is, it allows us to determine
an iron-containing species is diamagnetic, paramagnetic, or if it exhibits magnetic ordering. Typically, these
measurements also allow us to determine the ground spin state of a species of interest.
Field-dependent Mössbauer spectroscopy
is a specialized, highly powerful spectroscopic tool
that can provide a wealth of information such the oxidation state, the asymmetry of the electronic charge distribution
(electric field gradient), the relative energies of the spin sublevels of the ground state (zero-field splitting), and
the strength of the interaction between the 57Fe nuclear spins and the electronic spins (hyperfine structure) of all
iron sites contained by a chemical species of interest. This information can be extracted regardless of the nature
of the iron-containing species, that is, there are no Mössbauer-silent iron sites. Albeit the ability to only detect
a single isotope, in this case 57Fe, might seem a limitation, the widespread distribution of iron-containing enzymes
and the tremendous technological importance of this element makes Mössbauer spectroscopy the preeminent technique for
the characterization of iron species. An important feature of this technique is that it allows for the quantification
of all iron species in a sample. However, this analysis requires recording zero-field spectra at very low temperatures,
usually below 10 K. At higher temperatures the fraction of nuclei that exhibit a recoilless absorption and emission of
γ-ray photons might be different for different sites. Performing field- and temperature-dependent measurements
informs us on the magnetic behavior of all iron species present in the sample, that is, it allows us to determine
an iron-containing species is diamagnetic, paramagnetic, or if it exhibits magnetic ordering. Typically, these
measurements also allow us to determine the ground spin state of a species of interest.
 Such studies require the acquisition of a series of very low-temperature (usually 4.2 K) spectra collected
at varying magnetic field strengths and a series of temperature-dependent spectra recorded at a high-field
(usually the highest accessible field, which in our case is 8 T). A successful spectroscopic investigation
might require up to 20 spectra per sample. For these measurements liquid helium is used not only to maintain
the superconductivity of the magnet, but also to control the temperature of the sample space. While at 4.2 K the sample
is submerged in liquid helium, at higher temperatures the sample is cooled by a stream of helium gas. For our
instrument, an ideal sample should contain 40 - 60 µg of 57Fe corresponding to ~1 mg iron of natural abundance
for non-isotopically enriched samples. Even for these samples, recording a spectrum of a
suitable signal-to-noise ratio in an applied magnetic field will require up to a day of instrument time.
Therefore, the collection of an entire data-set might necessitate up to three weeks of instrument time per sample.
Since all this time the superconducting magnet needs to be kept cold and the sample in a stream of cold helium, cryogenic
liquid helium is a crucial requirement to field-dependent Mössbauer spectroscopy.
Such studies require the acquisition of a series of very low-temperature (usually 4.2 K) spectra collected
at varying magnetic field strengths and a series of temperature-dependent spectra recorded at a high-field
(usually the highest accessible field, which in our case is 8 T). A successful spectroscopic investigation
might require up to 20 spectra per sample. For these measurements liquid helium is used not only to maintain
the superconductivity of the magnet, but also to control the temperature of the sample space. While at 4.2 K the sample
is submerged in liquid helium, at higher temperatures the sample is cooled by a stream of helium gas. For our
instrument, an ideal sample should contain 40 - 60 µg of 57Fe corresponding to ~1 mg iron of natural abundance
for non-isotopically enriched samples. Even for these samples, recording a spectrum of a
suitable signal-to-noise ratio in an applied magnetic field will require up to a day of instrument time.
Therefore, the collection of an entire data-set might necessitate up to three weeks of instrument time per sample.
Since all this time the superconducting magnet needs to be kept cold and the sample in a stream of cold helium, cryogenic
liquid helium is a crucial requirement to field-dependent Mössbauer spectroscopy.