Synthetic Clusters
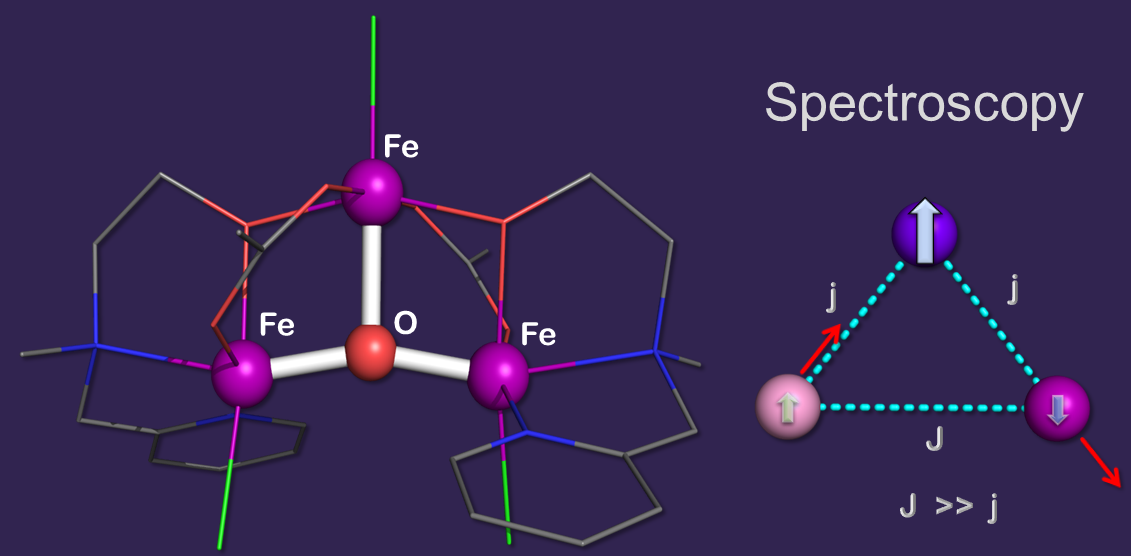 Polynuclear metal clusters comprised of oxo- and hydroxo-bridged high-spin ferric ions are widespread throughout mineralogy and biology.
Furthermore, some synthetic examples that have a nonzero ground-spin state have been shown to exhibit single molecule magnet (SMM) behavior;
that is, their magnetization exhibits slow relaxation at low temperatures. Consequently, the quest for high-spin iron(III) clusters
that exhibit unusual magnetic properties or function as enzymatic model complexes is an active area of research and, to date, has
led to the characterization of numerous polynuclear ferric complexes with interesting chemistry, spin topologies, magnetic, and
electronic structures. In biological systems, diferric clusters are abundant and often encountered in the resting state of nonheme
enzymes, such as the oxygen transport protein hemerythrin, purple acid phosphatase, ribonucleotide reductase, soluble
methane monooxygenase, and the ferroxidation site of ferritins. In contrast, trinuclear iron(III) such as the cluster shown here as an example are considerably less
common with only a handful of examples having been identified so far, such as the iron–sulfur clusters of ferredoxins and aconitase enzymes,
the oxo-bridged cluster of ferreascidin, and the ferroxidase site of some bacterial ferritins. This cluster comprises three high-spin
ferric ions and exhibits a T-shaped site topology. Our spectroscopic investigation revealed a S = 5/2 ground
state which originates from the dominant antiferromagetic coupling of two iron(III) sites. A theoretical analysis of the magnetic hyperfine structure
revealed the mixing of two excited states into the ground state which, in turn, is induced by a minor structural distortion.
Polynuclear metal clusters comprised of oxo- and hydroxo-bridged high-spin ferric ions are widespread throughout mineralogy and biology.
Furthermore, some synthetic examples that have a nonzero ground-spin state have been shown to exhibit single molecule magnet (SMM) behavior;
that is, their magnetization exhibits slow relaxation at low temperatures. Consequently, the quest for high-spin iron(III) clusters
that exhibit unusual magnetic properties or function as enzymatic model complexes is an active area of research and, to date, has
led to the characterization of numerous polynuclear ferric complexes with interesting chemistry, spin topologies, magnetic, and
electronic structures. In biological systems, diferric clusters are abundant and often encountered in the resting state of nonheme
enzymes, such as the oxygen transport protein hemerythrin, purple acid phosphatase, ribonucleotide reductase, soluble
methane monooxygenase, and the ferroxidation site of ferritins. In contrast, trinuclear iron(III) such as the cluster shown here as an example are considerably less
common with only a handful of examples having been identified so far, such as the iron–sulfur clusters of ferredoxins and aconitase enzymes,
the oxo-bridged cluster of ferreascidin, and the ferroxidase site of some bacterial ferritins. This cluster comprises three high-spin
ferric ions and exhibits a T-shaped site topology. Our spectroscopic investigation revealed a S = 5/2 ground
state which originates from the dominant antiferromagetic coupling of two iron(III) sites. A theoretical analysis of the magnetic hyperfine structure
revealed the mixing of two excited states into the ground state which, in turn, is induced by a minor structural distortion.
Stoian SA, Peng YR, Beedle CB, Chung Y-J, Lee G-H, En-Che Y, Hill S; Inorg. Chem. 2017, 56, 10861-10874
 Hydrogenases are bacterial enzymes that catalyze the reversible interconversion of H2 into protons and electrons, producing H2(g) efficiently through an
environmentally benign process. Hydrogenases employ first-row transition metals, such as iron (and nickel) to effect homogeneous hydrogenesis,
which occurs at low potentials, thus demanding iron in very low oxidation states. The active site of the [FeFe] hydrogenases, known as
the H-cluster, is a six-iron structure in which an [Fe4S4]2+ cubane is connected through a cysteinyl bridge to an organometallic
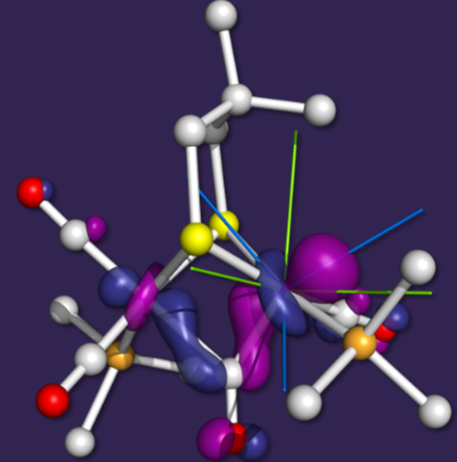
dinuclear subcluster, [Fe2]H (the catalytic site where dihydrogen is produced or oxidized). The figure on the left shows the oxidized form, [FeIFeII],
of a model complex of [Fe2]H and its highest occupied molecular orbital (HOMO). Such models are crucial in elucidating the behavior and properties of the enzymatic active stie.
A combined spectroscopic and theoretical analysis of this cluster allowed us to rationalize its unusual electronic and structural features and to correlate them with that of the enzyme.
Thus, the Fe–Fe bond of the reduced form, [FeI2], involves two dz2-type orbitals and is crucial in keeping the structure intact in the presence of strain.
Upon oxidation, the distal iron site is not restricted by the Fe–Fe bond any longer, and thus the more stable isomer results from inversion of the square pyramid, rotating the dz2 orbital of FeI and the formation of
a 2-electrons three-centers bond shown here.
Hydrogenases are bacterial enzymes that catalyze the reversible interconversion of H2 into protons and electrons, producing H2(g) efficiently through an
environmentally benign process. Hydrogenases employ first-row transition metals, such as iron (and nickel) to effect homogeneous hydrogenesis,
which occurs at low potentials, thus demanding iron in very low oxidation states. The active site of the [FeFe] hydrogenases, known as
the H-cluster, is a six-iron structure in which an [Fe4S4]2+ cubane is connected through a cysteinyl bridge to an organometallic
dinuclear subcluster, [Fe2]H (the catalytic site where dihydrogen is produced or oxidized). The figure on the left shows the oxidized form, [FeIFeII],
of a model complex of [Fe2]H and its highest occupied molecular orbital (HOMO). Such models are crucial in elucidating the behavior and properties of the enzymatic active stie.
A combined spectroscopic and theoretical analysis of this cluster allowed us to rationalize its unusual electronic and structural features and to correlate them with that of the enzyme.
Thus, the Fe–Fe bond of the reduced form, [FeI2], involves two dz2-type orbitals and is crucial in keeping the structure intact in the presence of strain.
Upon oxidation, the distal iron site is not restricted by the Fe–Fe bond any longer, and thus the more stable isomer results from inversion of the square pyramid, rotating the dz2 orbital of FeI and the formation of
a 2-electrons three-centers bond shown here.
Stoian SA, Hsieh CH, Singleton ML, Casuras AF, Darensbourg MY, McNeely K, Sweely K, Popescu CV; J. Biol. Inorg. Chem. 2013, 18, 609-622
Biological Clusters
 Bacterial synthesis of diesel-length alkanes and alkenes expands the biofuel inventory beyond alcohols and esters by making a source of renewable liquid fuels.
Recently, a two-gene cluster required for alkane/alkene biosynthesis was identified in cyanobacteria. The hydrocarbons formed had an odd chain length (C13, C15, and C17),
indicating they were biosynthesized by removal of the C-1 carbon of even-chain-length fatty acyl groups. In this biosynthetic pathway, the fatty acyl group is reduced by an
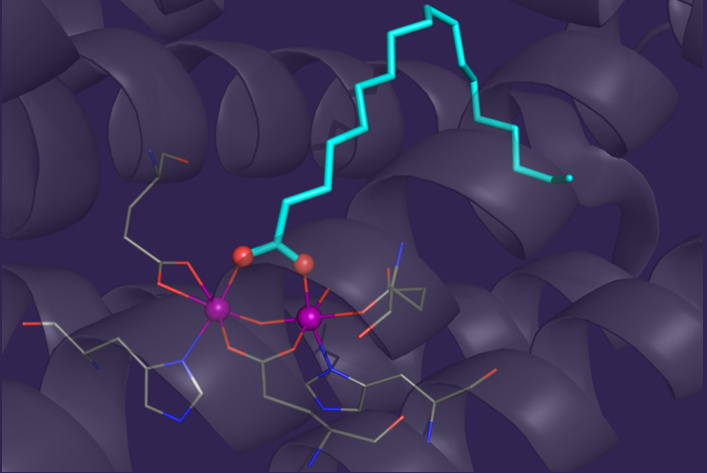
acyl-ACP reductase, a member of the short-chain dehydrogenase family, to produce the corresponding aldehyde. The second enzyme in the pathway, a dinuclear iron-cluster-containing
enzyme (shown here), removes the aldehydic carbon to produce a hydrocarbon. Initially, the displaced single-carbon product was proposed to be carbon monoxide, on the basis of previous reports
on similar reactions observed in whole cells and cell extracts of plants and animals. More recently, formate was shown to be the coproduct of the hydrocarbon-generating
enzyme, with the second oxygen atom derived from atmospheric dioxygen. In light of this, the hydrocarbon-generating enzyme has been given the name aldehyde-deformylating
oxygenase (ADO). The observation of alcohol and aldehyde products derived from the initially formed alkane product
suggests a reactive species similar to that formed by methane monooxygenase (MMO) and other members of the bacterial
multicomponent monooxygenase family. Accordingly, characterization by EPR and Mössbauer spectroscopies shows that the
electronic structure of the ADO cluster is similar, but not identical, to that of the MMO hydroxylase component. In particular,
the two irons of ADO reside in nearly identical environments in both the oxidized and fully reduced states, whereas those of
MMOH show distinct differences.
Bacterial synthesis of diesel-length alkanes and alkenes expands the biofuel inventory beyond alcohols and esters by making a source of renewable liquid fuels.
Recently, a two-gene cluster required for alkane/alkene biosynthesis was identified in cyanobacteria. The hydrocarbons formed had an odd chain length (C13, C15, and C17),
indicating they were biosynthesized by removal of the C-1 carbon of even-chain-length fatty acyl groups. In this biosynthetic pathway, the fatty acyl group is reduced by an
acyl-ACP reductase, a member of the short-chain dehydrogenase family, to produce the corresponding aldehyde. The second enzyme in the pathway, a dinuclear iron-cluster-containing
enzyme (shown here), removes the aldehydic carbon to produce a hydrocarbon. Initially, the displaced single-carbon product was proposed to be carbon monoxide, on the basis of previous reports
on similar reactions observed in whole cells and cell extracts of plants and animals. More recently, formate was shown to be the coproduct of the hydrocarbon-generating
enzyme, with the second oxygen atom derived from atmospheric dioxygen. In light of this, the hydrocarbon-generating enzyme has been given the name aldehyde-deformylating
oxygenase (ADO). The observation of alcohol and aldehyde products derived from the initially formed alkane product
suggests a reactive species similar to that formed by methane monooxygenase (MMO) and other members of the bacterial
multicomponent monooxygenase family. Accordingly, characterization by EPR and Mössbauer spectroscopies shows that the
electronic structure of the ADO cluster is similar, but not identical, to that of the MMO hydroxylase component. In particular,
the two irons of ADO reside in nearly identical environments in both the oxidized and fully reduced states, whereas those of
MMOH show distinct differences.
Aukema KG, Makris TM, Stoian SA, Richman JE, Münck E, Lipscomb JD, Wackett LP; ACS Catal. 2013, 3, 2228–2238