3d5-8 Ions with a High-Spin Configuration and Square-Planar Geometry
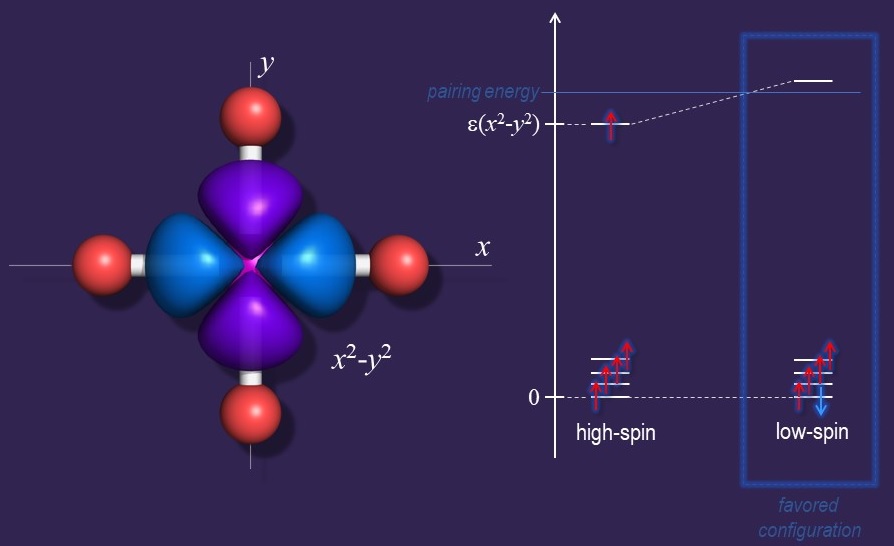 Unusual compounds can have unusual electronic structures. Investigating such cases enriches our understanding of bonding and chemical reactivity. One such example is
represented by high-spin, square-planar complexes of d5-8 ions.
Unusual compounds can have unusual electronic structures. Investigating such cases enriches our understanding of bonding and chemical reactivity. One such example is
represented by high-spin, square-planar complexes of d5-8 ions.
In particular, iron complexes of this sort are unusually rare, fewer than ten examples having been characterized to date.
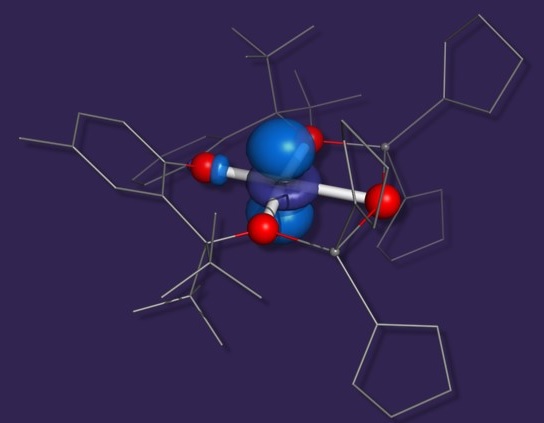
For most of the cases reported up to date, the stabilization of square-planar geometry remains puzzling. We are currently investigating a series of high-spin, square-planar complexes of Co(II) and Fe(II) ions. The intricate factors responsible for their stabilization are yet to be elucidated.
Pascualini ME, Di Russo NV, Thuijs AE, Ozarowski A, Stoian SA, Abboud KA, Christou G, Veige AS Chem. Sci., 2015, 6, 608-612;
Pascualini M, Stoian SA, Ozarowski A, DiRusso N, Thuijs A, Khalil A, Christou G, Veige AS Dalton Trans., 2015, 44, 20207-20215;
Pascualini ME, Stoian, SA,Ozarowski, A, Abboud, KA, Veige, AS Inorg. Chem. , 2016, 55, 5191-5200
Low-Coordinate Compounds with Unquenched Orbital Momenta
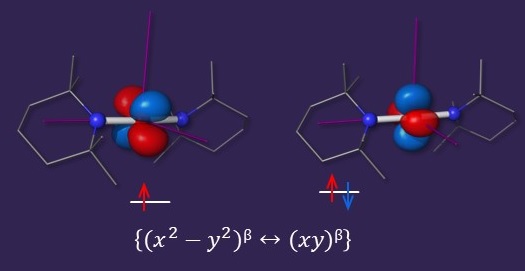
Among low-coordinate complexes, two-coordinate iron(II) compounds are some of the most interesting. These compounds not only exhibit SMM-like behavior due to the quasi-degeneracy of the {x2-y2 ↔ xy} orbitals but also a fairly large pseudo Jahn-Teller effect. These features allow us to evaluate the interplay between the unquenching of angular momentum and vibronic coupling.