Spin-Crossover Molecules
 Some systems can switch reversibly between two states. This behavior is known as bistability. For molecules,
such switching is usually accompanied by a dramatic inflection in their magnetic, structural, and optical properties.
Molecular moieties exhibiting this behavior have potential of functioning as single-molecule bits in data storage
devices, as sensitive (temperature, pressure, and light) sensors, and optical and magnetic indicators (due to their
dramatic changes in colour and magnetic moment). For example, Fe(II) d6 ions can exhibit both high-spin
(HS, S = 2) and low-spin (LS, S = 0) electronic configurations.
The
largest fraction of known SCO compounds is accounted by quasi-octahedral Fe(II) ions supported by N6 coordination
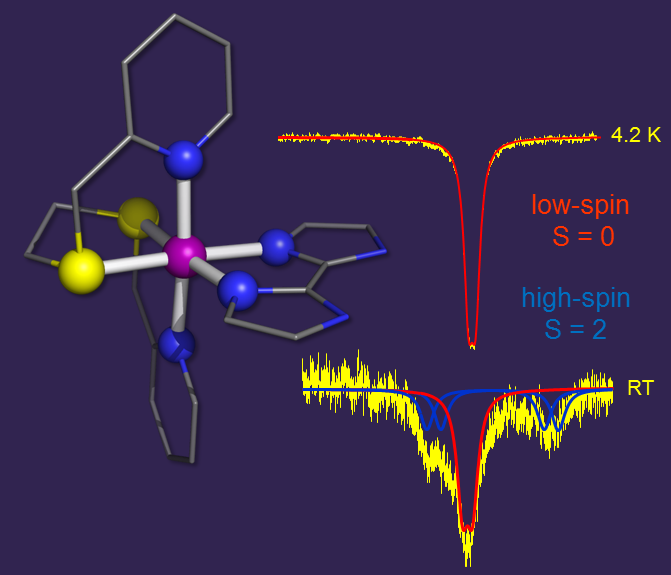
environments. Shown on the left is an example of a rare family of SCO complexes featuring a N4S2 environment.
The temperature-induced SCO behavior of this compound is reflected by the difference between the zero-field Mössbauer spectra recorded at
4.2 K and room temperature.
Some systems can switch reversibly between two states. This behavior is known as bistability. For molecules,
such switching is usually accompanied by a dramatic inflection in their magnetic, structural, and optical properties.
Molecular moieties exhibiting this behavior have potential of functioning as single-molecule bits in data storage
devices, as sensitive (temperature, pressure, and light) sensors, and optical and magnetic indicators (due to their
dramatic changes in colour and magnetic moment). For example, Fe(II) d6 ions can exhibit both high-spin
(HS, S = 2) and low-spin (LS, S = 0) electronic configurations.
The
largest fraction of known SCO compounds is accounted by quasi-octahedral Fe(II) ions supported by N6 coordination
environments. Shown on the left is an example of a rare family of SCO complexes featuring a N4S2 environment.
The temperature-induced SCO behavior of this compound is reflected by the difference between the zero-field Mössbauer spectra recorded at
4.2 K and room temperature.
Yergeshbayeva S, Hrudka JJ, Lengyel J, Erkasov R, Stoian SA, Dragulescu-Andrasi A, Shatruk M Inorg. Chem., 2017,56, 11096-11103
Single-Molecule Magnets
Some molecules can behave like tiny, individual magnets. Hence, we call them single-molecule magnets (SMM). One major current research goal is to design SMMs with high blocking temperatures. Such compounds can function as qubits (quantum bits) and have the potential to revolutionize the way we store and process digital information. Unlike for bulk conventional magnets the hysteretic behavior of single-molecule magnets does not rely on long-range magnetic ordering and is of purely molecular origin.
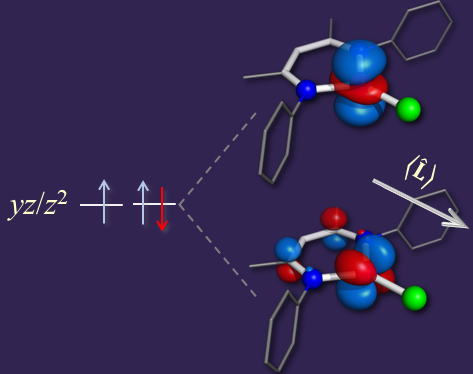
The figure on the right shows a β-diketiminate supported, heteroleptic iron(II) complex, LFeIIX, which exhibits a magnet-like behavior. In this case, the two ligands enforce a planar, trigonal geometry around the iron(II) ion. This environment leads to the z2/yz orbitals being non bonding orbitals and virtually degenerate. This orbital set accommodates two α, spin-up electrons and only one β, spin-down electrons. The two orbital states corresponding to the β electron occupying either the z2 or the yz orbital are mixed by the spin-orbit coupling which leads to an unquenched orbital momemntum along x, that is, along the FeX bond. In turn, this electronic structure leads to a slow relaxation of the magnetization and magnetic bistability.
 We have often encountered a SMM-like behavior for β-diketiminate supported iron(II) complexes.
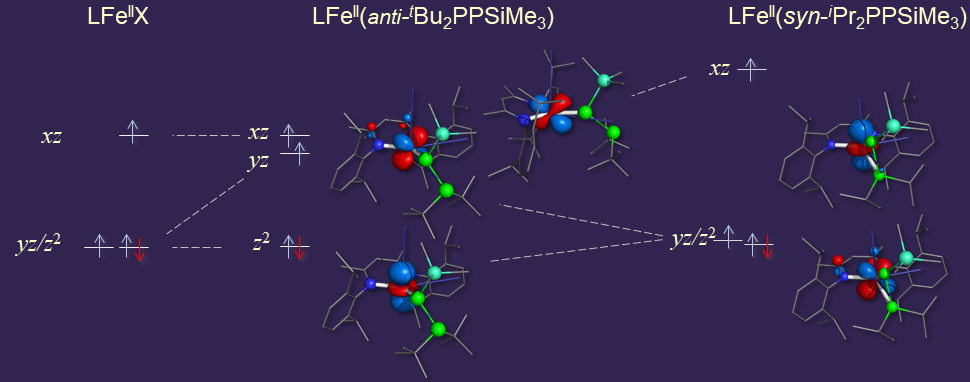
Most recently we have observed yz/z2-type orbital degeneracy for a novel class of [LFeII(phosphanylphosphido)] complexes.
The structural and spectroscopic characterization of these compounds revealed that their electronic structures and magnetic properties are tightly coupled to
the conformation of the phosphanylphosphido ligands. The change from an anticlinal to a synclinal conformation of the R2PPSiMe3- moiety leads not only to an
increase in the coordination number but also to a change in the nature of the electronic ground state and the character of spin−orbit interactions.
We have often encountered a SMM-like behavior for β-diketiminate supported iron(II) complexes.
Most recently we have observed yz/z2-type orbital degeneracy for a novel class of [LFeII(phosphanylphosphido)] complexes.
The structural and spectroscopic characterization of these compounds revealed that their electronic structures and magnetic properties are tightly coupled to
the conformation of the phosphanylphosphido ligands. The change from an anticlinal to a synclinal conformation of the R2PPSiMe3- moiety leads not only to an
increase in the coordination number but also to a change in the nature of the electronic ground state and the character of spin−orbit interactions.
Grubba R, Kaniewska K, Ponikiewski Ł, Cristóvão B, Ferenc W, Dragulescu-Andrasi A, Krzystek J, Stoian SA, Pikies, J
Inorg. Chem., 2017, 56, 11030-11042